A frozen biopsy, commonly referred to as frozen section analysis, is a rapid diagnostic procedure widely used during surgeries to provide real-time information about a patient’s tissue sample. This method plays a vital role in determining the nature of a lesion—benign or malignant—and helps surgeons make immediate decisions during operative procedures. The use of advanced cryostat microtomy, histopathological staining, and intraoperative consultation makes frozen biopsy an indispensable component of modern medical practice.
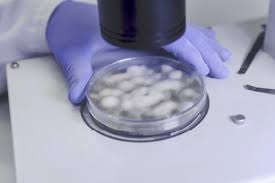
Frozen biopsy involves a highly sophisticated process. Once the surgeon removes a small portion of tissue during an operation, it is immediately transported to the pathology laboratory. The sample is then embedded in a medium, typically optimal cutting temperature compound (OCT), and rapidly frozen using a cryostat machine. A cryostat is a specialized apparatus that maintains extremely low temperatures, usually between –20°C and –30°C, allowing the tissue to harden sufficiently for sectioning.
The hardened tissue is then finely sliced using a microtome blade, producing thin sections usually 5–10 micrometers thick. These sections are mounted on glass slides and stained using rapid staining techniques such as hematoxylin and eosin (H&E). The stained slides are examined under a microscope by a surgical pathologist, who evaluates features such as cellular morphology, nuclear atypia, mitotic activity, and architectural distortion.
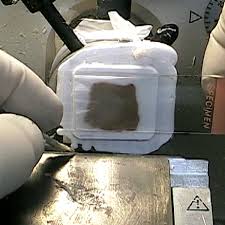
One primary advantage of frozen biopsy is the speed of diagnosis. The entire procedure—from tissue removal to microscopic interpretation—typically takes 10 to 20 minutes. This real-time turnaround helps surgeons decide whether to proceed with more extensive surgery, such as complete tumor excision, lymph node dissection, or margin clearance. For example, during breast cancer surgery, frozen section analysis determines whether the resection margins are free of malignant cells, ensuring complete tumor removal.
Frozen biopsy also plays an essential role in identifying metastatic involvement of lymph nodes, especially in cancers of the thyroid, breast, and gastrointestinal tract. It helps surgeons assess the extent of disease spread, which significantly impacts the overall treatment plan.

Despite its many benefits, frozen biopsy has limitations. The rapid freezing process can sometimes cause ice crystal artifacts, which may distort cellular structures and reduce diagnostic clarity. Certain tissue types, such as fatty tissue or those requiring special stains and immunohistochemistry, are not ideally suited for frozen section analysis. In such cases, a more detailed formalin-fixed, paraffin-embedded (FFPE) examination is recommended for final confirmation.
Accuracy in frozen biopsy relies heavily on the expertise of the pathologist and proper tissue sampling. Errors in interpretation, although rare, may occur due to sampling bias or artifact-related distortions. Therefore, frozen section results are always correlated with permanent section histopathology for a definitive diagnosis.
In modern clinical practice, frozen biopsy remains an invaluable diagnostic tool, bridging the gap between surgical decision-making and pathology. Its rapidity, precision, and ability to guide intraoperative strategy contribute significantly to improved patient outcomes and reduced surgical complications. As advancements in digital pathology and imaging techniques continue, frozen biopsy is expected to become even more refined, enhancing its role in real-time surgical diagnostics.
Understanding Frozen Biopsy: A Critical Diagnostic Tool in Modern Surgical Pathology
A frozen biopsy, commonly referred to as frozen section analysis, is a rapid diagnostic procedure widely used during surgeries to provide real-time information about a patient’s tissue sample. This method plays a vital role in determining the nature of a lesion—benign or malignant—and helps surgeons make immediate decisions during operative procedures. The use of advanced cryostat microtomy, histopathological staining, and intraoperative consultation makes frozen biopsy an indispensable component of modern medical practice.
Frozen biopsy involves a highly sophisticated process. Once the surgeon removes a small portion of tissue during an operation, it is immediately transported to the pathology laboratory. The sample is then embedded in a medium, typically optimal cutting temperature compound (OCT), and rapidly frozen using a cryostat machine. A cryostat is a specialized apparatus that maintains extremely low temperatures, usually between –20°C and –30°C, allowing the tissue to harden sufficiently for sectioning.
The hardened tissue is then finely sliced using a microtome blade, producing thin sections usually 5–10 micrometers thick. These sections are mounted on glass slides and stained using rapid staining techniques such as hematoxylin and eosin (H&E). The stained slides are examined under a microscope by a surgical pathologist, who evaluates features such as cellular morphology, nuclear atypia, mitotic activity, and architectural distortion.
One primary advantage of frozen biopsy is the speed of diagnosis. The entire procedure—from tissue removal to microscopic interpretation—typically takes 10 to 20 minutes. This real-time turnaround helps surgeons decide whether to proceed with more extensive surgery, such as complete tumor excision, lymph node dissection, or margin clearance. For example, during breast cancer surgery, frozen section analysis determines whether the resection margins are free of malignant cells, ensuring complete tumor removal.
Frozen biopsy also plays an essential role in identifying metastatic involvement of lymph nodes, especially in cancers of the thyroid, breast, and gastrointestinal tract. It helps surgeons assess the extent of disease spread, which significantly impacts the overall treatment plan.
Despite its many benefits, frozen biopsy has limitations. The rapid freezing process can sometimes cause ice crystal artifacts, which may distort cellular structures and reduce diagnostic clarity. Certain tissue types, such as fatty tissue or those requiring special stains and immunohistochemistry, are not ideally suited for frozen section analysis. In such cases, a more detailed formalin-fixed, paraffin-embedded (FFPE) examination is recommended for final confirmation.
Accuracy in frozen biopsy relies heavily on the expertise of the pathologist and proper tissue sampling. Errors in interpretation, although rare, may occur due to sampling bias or artifact-related distortions. Therefore, frozen section results are always correlated with permanent section histopathology for a definitive diagnosis.
In modern clinical practice, frozen biopsy remains an invaluable diagnostic tool, bridging the gap between surgical decision-making and pathology. Its rapidity, precision, and ability to guide intraoperative strategy contribute significantly to improved patient outcomes and reduced surgical complications. As advancements in digital pathology and imaging techniques continue, frozen biopsy is expected to become even more refined, enhancing its role in real-time surgical diagnostics.

















